Temperature Mapping
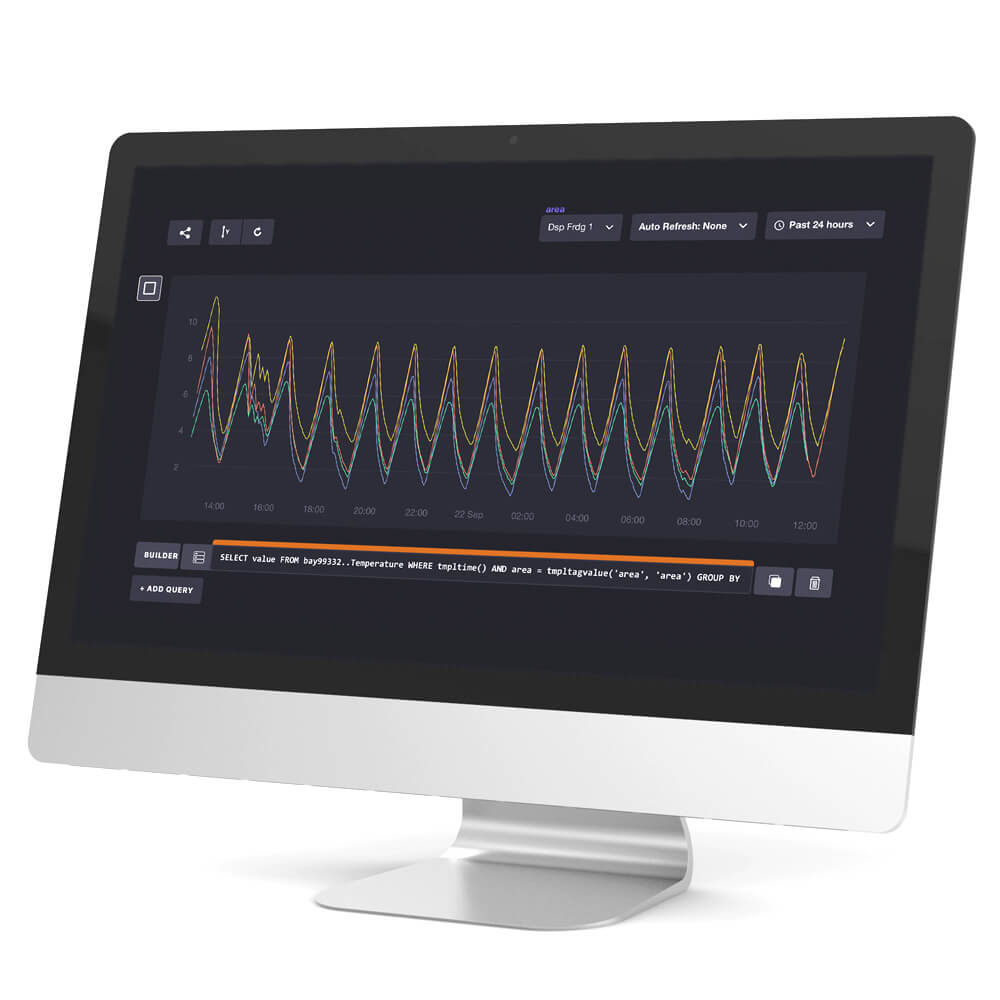
We do comprehensive temperature mapping studies of your storage areas based on strict WHO guidelines.
Why do a Mapping Study?
A temperature mapping exercise is legally required for any space allocated for the storage and handling of pharmaceutical products with a specified labelled storage temperature. A mapping study establishes the temperature distribution within the area being mapped.
The data collected thus provides an essential source of information to ensure that all products can be safely and correctly stored within their labelled temperature range.
Important considerations
- Legal requirement
- Essential information
- Product safety
Temperature Mapping provides certainty.
In an environment of escalating competition, tighter margins and growing costs associated with an expanding regulatory burden, maintaining high standards of pharmaceutical related practices and services pose a number of challenges.
In addition, responsible parties are faced with providing reliable up to date evidence of compliance, in the case of audits and investigations by the South African Pharmacy Council (SAPC) and other stakeholders, with numerous rules relating to Good Pharmacy Practice (GPP).